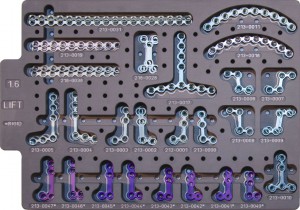
1. OsteoMed Cranio and Maxillfacial Fixation System(product name:Fast-FlapTM)
Product standard:
Import product standard YZB/USA 6125-2012《Cranio and Maxillfacial Fixation System》
Struture and performance:
It is composed of metal plates and metal screw, uses Ti6A14V ELI titanium alloy materials that meets the standard of ASTM F136. Product surface is without color. Non sterilization condition.
Scope of application:
The product is suitable for cranial and maxillofacial bone trauma or assisting fixation for reconstruction.
2.Cranial and maxillofacial trauma fixation system(product name:OsteoMed CFxTM)
Product standard:
Import product register standard YZB/USA 6126-2012 《Cranial and maxillofacial truma fixation system》
Struture and performance:
The product has metal plates, titanium mesh and metal screw. Metal screw and partial metal plates is made of Ti6A14V ELI titanium alloy materials according to the standard of ASTM F136, titanium mesh and partial metal plates is made of pure titanium material which meets the standard of ASTM F67, details as shown in the product size specification list. Product surface is without color. Non sterilization condition.
Scope of application:
This product is suitable for the skull fracture, facial trauma fracture reconstruction.
3.Cranial and maxillofacial plate fixation system(product:OSA System TM)
Product standard:
YZB/USA 6512-2012″Cranial and maxillofacial plate fixation system”
Struture and performance:
Product is composed of metal plates and metal screw, metal plates use grade 2 pure titanium in accordance with ASTM F – 67 , the screw use Ti6A14V ELI titanium alloy materials that meets the standard of ASTM F136 . Product surface is without color. Non sterilization packaging.
Scope of application:
This product is applicable to the midface, skull trauma, craniofacial surgery, reconstructive surgery, and the jaw surgery for upper and lower jaw.
4.Cranial and maxillofacial fixation system [M3 trauma Fixation System(Anodized)]
Product standard:
YZB/USA 0926-2014″Cranial and maxillofacial fixation system”
Struture and performance:
The production is composed of cranial and maxillofacial fixation plate and screw, the fixed plate is made of grade 1, grade 2, grade 3 and grade 4 pure titanium material that meets the ISO5832-2 standard ; the screw adopt Ti6A14V titanium alloy material that meets the appropriate ISO5832-3 standard, specific list as shown in the specifications, the pure titanium and titanium alloy products surface is treated by anodic oxidation. Non sterilization packaging.
Scope of application:
Suitable for cranial and maxillofacial fracture internal fixation.
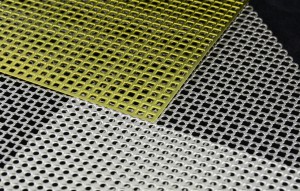
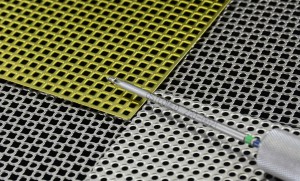
5。Titanium mesh【M3 mesh trauma Fixation System(Anodized)]
Product standard:
YZB/USA 0924-2014《Titanium mesh》
Struture and performance:
It is made of grade 1, grade 2, grade 3 and grade 4 pure titanium material that meets the standard of ISO5832-2. Details as shown in the specifications list, the surface is treated by anodic oxidation . Non sterilization package.
Scope of application:
Suitable for cranial and maxillofacial fracture internal fixation.
Contact US
Contact number:020-34093832
E-mail:t-med003@foxmail.com
9:30 to 6:00 pm on weekdays, please contact us, please send a message at other times.
Contact number:020-34093832
E-mail:t-med003@foxmail.com
9:30 to 6:00 pm on weekdays, please contact us, please send a message at other times.